| Dose Ratio mcg/kg | Weight in lbs. | Weight in kg | Dose in mcg | Dose in mg | Dose in ml |
|---|---|---|---|---|---|
| 200 | |||||
| 400 | |||||
| 800 | |||||
| 1200 | |||||
Research Regarding Ivermectin, Fenbendazole & Mebendazole
Using The Search Bar
The "Search Bar" is divided into three sections: 1 - The search argument section; 2 - The search submit button; 3 - The search site selection dropdown.
The current (hidden) search engine, that is hard coded, is the Bing.com/search site. This will eventually change to allow selection of different search engine.
The "site selection" dropdown allows the selection of several different sites on which to focus the search argument.
The search argument can be tailored to your specific needs. As an example your search argument will end up looking something like "site:nih.gov cancer vitamin c prostate." You can select another site and try your argument again and get different results.
I'm providing this tool, because it eliminates a lot of the SPOAFStating Personal Opinion As Fact websites that are popping up, and are filled with ads, and are just there to make money off the ads.
On some some sites you may have to reply to the simple CAPTCHA to continue. It's their way to prevent BOTSBOTS is short for roBOTSF7-1 from doing massive searches.
If you would like to format your own search argument for a different site here is an example you can use: (it will require you to respond to the CAPTCHA.)
DISCLAIMER:THIS PAGE IS NOT INTENDED TO BE MEDICAL ADVICE!
It is infomation from various sources (as referenced), and may be formatted in a more understandable way.
The mention of any article, book, or document does not imply an endorsement of, or concurrence with those items.
Hovering over words, or phrases, that have a background color will display more information.
Dosage CalculatorTopDo NOT use the information on this page as medical advise!
CAUTION! If you have alpha-gal syndrome, CONSIDER WELL, NOT TAKING THE IVERMECTIN MENTIONED ON THIS PAGE, except for the Ivermectin that is alpha-gal safe!
It could be very dangerous to your health!
The data on this page is gathered from various sources and presented here for your use.
Propylene Glycol in an ingredient in the both products mentioned on this page, so if there is a chance you might have AGS, you should consider NOT taking these products just to get the Ivermectin
Please contact me if you find data that is incorrect.
Enter your weight in pounds in the yellow box. Then click (or tap) on the calculate button.
Caution - The dosage in pink is not recommended without the advice of a doctor!
There are predominantly two dosage ratios (200mcg/kg and 400mcg/kg) mentioned in many papers3, 18, 43. The 200 mcg/kg is a prophylactic (intended to prevent disease) and the 400 mcg/kg is an aggressive dose intended to treat a disease. Note: mcg is micrograms.
The aggressive dose may be administered orally once per day for 4 days. The first daily dose will put a percentage of the peak level of that dose into your system within a few minutes. The level in your system will then decline. At about 18 hours the level in your system will decline to 50% of the peak dose, and will continue to decline until you take the next dose.
When you take the next dose, the same effect happens, but this time the peak dose will be added to the residual that is left in your system from the previous dose(s). This same thing will happen after each dose.
The limit of the peaks will be reached after the 4th dose, which should be your last dose of the "agressive" dosage for the week.
Drug Half-Life CalculatorTop
Following is a Half-Life Calculator where you can enter your calculated dose (from above). Enter the half-life
- Ivermectin = 18 hours
- Fenbendazole = 8 hours
- Mebendazole = 3 to 6 hours
- This can vary greatly based on the amount of fat consumed with the dose.
- It can also vary significantly based on the saponin level in the food consumed at the same time.
Input VariablesTop
You can Change this (default) dose and the graph will change dynamically.
This percentage is explained in Science Direct half-life info.
If taken regularly
If taken once
Sources of IvermectinTop
I have stopped taking the Durvet product since I was diagnosed with alpha-gal.
I am now taking a compounded 20 mg dose daily.
I was prescribed the Ivermectin by my doctor and got the prescription filled by CareFirst Specialty Pharmacy.
- DurvetImageThe brand I was using
- Ingredients
- Ivermectin
- Molecular Formula: C48H74O14
- Molecular Weight: 875.09
- Glycerol Formal
- Molecular Formula: C4H8O3
- Formula Weight: 104.1
- Propylene Glycol
- Generally considered Safe by the FDA.
- An article written about the use and safety
- Noromectrin (Green Label)I do NOT recommended this product for human consumption!
- Ingredients
- Ivermectin
- Clorsulon
- Propylene Glycol
- Sourced from India company
- Horse Paste from Various Sources:
- The normal dose of ivermectin for deworming a horse is typically 200 micrograms (µg) per kilogram (kg) of body weight. This translates to about 91 micrograms per pound (lb). Note that 200 µg/kg is the same as the calculator uses as a prophylactic dose.
- For most commercial ivermectin paste formulations for horses, which are usually at a concentration of 1.87%, one syringe generally contains enough paste to treat a horse weighing up to 1250 pounds at this recommended dose. The syringe is calibrated with markings for every 250 pounds of body weight, allowing for accurate dosing based on the horse's weight.
- Here's how it works:
- Each notch on the syringe typically delivers enough paste to treat 250 lbs of horse body weight.
- If your horse weighs 1000 pounds, you would adjust the plunger to the 1000-pound mark or the fourth notch from the bottom.
- If your horse only weighs 125 pounds, you would only use 1/2 of one notch.
- It's important to ensure you get the horse's weight as accurately as possible, either by using a weight tape or a scale, to avoid underdosing or overdosing. Underdosing can lead to ineffective treatment, while overdosing can cause toxicity, though ivermectin has a relatively wide safety margin in horses when used appropriately.
- The bottom line is: Know what you're doing before you do it!
Sold at Tractor Supply
I purchased the 250ml bottle for $79.99 and expect it to last at least a year.
That puts my cost at $0.32 USD per ml. or $0.64 USD per dose for my weight.
For people that have alpha-gal (α-Gal) sensitivity, you should be aware that glycerol (glycerin) can be sourced from both plants and animals57
I believe the Clorsulon in this product represents a significant danger to those who have any sensitivity to sulfonamide drugs.
I am still researching this product. I will update as I gather information.
This product is sold at my local MFA
Caution! This ingredient may be potentially dangerous!
The Human Metabolome Database (HMDB) describes Clorsulon:
Also known as curatrem, Clorsulon belongs to the class of organic compounds known as aminobenzenesulfonamides
In 2014 the Committee for Medicinal Products for Veterinary Use (CVMP) in the EU set a Minimum Residue Limit (MRL) in bovine milk29.
Caution! Clorsulon is classified as a sulfonamide and is capable of causing anaphylactic shock32.
Article indicates 7mg/kg in sheep
Biological Half-Life of Clorsulon
Generally considered Safe by the FDA.
Ivermectin Tablet - Uses, Side Effects, Composition & Price This is a website in India with info that can be used to comparison shop.
Shop around and you can find IVM (Ivermectin) as low as $.02 USD per 12mg tablet.
The chart, shown at the "pacehospital" website, indicates the price can vary from about $0.30 to $4.07 per dose.
Update: I contacted supplier in India and the cost for 1 box (200 12mg tablets) with shipping and customs would be $89.00 USD. That's $0.44 per dose.
Information About IvermectinTop
- CDC says it should be used for scabies and worms.1
- May shorten duration of Covid-19.2, 6
- May participate in the war against cancers (prostate, breast, ovarian, leukemia)3, 55
- In vitro and in vivo antitumor activities of ivermectin.4
- Ivermectin-induced drug reaction5, 6
- Associated with lower Covid-19 mortality.7
- A new tool to eliminate malaria?27
- A possible weapon against West Nile Virus?26
- Nobel prizes awarded for discovery and practical use of Ivermectin. 12, 40
- South Carolina Dept. of Health tries to bury the truth of Ivermectin. 39
- 2023 study finds IVM not effective as COVID treatment45
- 2019 FDA approved 1% Ivermectin for treatment of rosacea in adults.53, 54,
- 2024 Ivermectin shows promise of improved Covid survival rates.56
- 2024 Ivermectin may have therapeutic benefits for IPF.58
- 2023 Eprinomectin: a derivative of ivermectin suppresses growth and metastatic phenotypes of prostate cancer cells59
- 2023 FDA capitulates on some Ivermectin positions and comments60
- 2021 Ivermectin is known to inhibit the replication of flavivirus.61
- 2019 Significant reduction in bed bug population after insects feed on blood of person that has taken only 1 dose of Ivermectin.62
- 2024 Mosquitocidal effect of ivermectin-treated nettings and sprayed walls on Anopheles gambiae s.s.63
- 2011 Ivermectin, ‘Wonder drug’ from Japan: the human use perspective.64
- 2022 Usual safe medication doses of Ivermectin drug in human beings is 0.2–0.4 mg/kg.65
- Jan. 2014 Human Demodex Mite: The Versatile Mite of Dermatological Importance66
- 2014 Severe clinical manifestation of demodex mites may require Ivermectin.67
- Dec. 2020 A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness68
- September 2019; Anti-parasitic Drug Ivermectin Exhibits Potent Anticancer Activity Against Gemcitabine-resistant Cholangiocarcinoma In Vitro69
- 2021 May 14; Ivermectin effectively inhibits hepatitis E virus replication, requiring the host nuclear transport protein importin α170
- 2012 Apr 25; Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug71
- 2018 Feb 20 In vitro activity of ivermectin against Staphylococcus aureus clinical isolates89
- Personal
- After the first dose, I experienced slight symptoms of vertigo and a short period (about an hour) of very slightly blurred vision (not anywhere near like removing my glasses).
- Dose 2, 3 and 4 on days 2, 3 and 4, each precipitated similar vertigo symptoms, although with shorter and less intense symptoms each successive day.
- My 5th dose was taken 168 hours (1 week) after number 4 dose and there were no side effects experienced.
- Dose #6 was taken 168 hours after dose #5. No side effects noted.
- Case studies
- Report of DRESS symptoms5. This report warrants discussion and investigation into the "what" was going on, and "why" was it happening. When there are just a few cases like this, after over 2 billion administrations of Ivermectin, they need to be thouroughly understood.
References:Top
- Top
- Top
- Top
- Top
- Top
- Top
- Top
- A fancy title to say: How to safely use Ivermectin to control mosquitos.
- In humans, studies with radio-labelled ivermectin show that peak plasma concentration of metabolites is about twice that of the parent drug and occurs later, at 7 h (vs four for parent drug). Plasma metabolites are less polar than the parent drug and could be fatty acid ester conjugates of the monosaccharides or aglycone of the parent drug.
- The plasma half-life of metabolites is about 72 h, fourfold that of the parent drug.
- Top
- Top
- Top
- Top
- Top
- Top
- The highest dose of ivermectin that we have used in our study is 12 mg (200 µg/kg) once daily for 5 consecutive daysNote: This dose is calculated for a 133 pound person., which resulted in a significantly shorter time for viral clearance. Viral clearance was earlier in the 5-day ivermectin treatment arm when compared with the placebo group (9.7 days vs 12.7 days; p = 0.02), but this was not the case for the ivermectin + doxycycline arm (11.5 days; p = 0.27). Similarly, clinical trials with a 3-day, once-daily dose of 400 µg/kg oral ivermectin in dengue patients has been shown to be safe, with accelerated clearance of the dengue virus nonstructural protein 1
- Top
- Top
- Top
- Top
- Top
- Top
- Top
- Top
- Top
- Type 1 hypersensitivity is IgE-mediated and may include an anaphylactic sequelae. (Interpretation: Anaphylactic shock is possible.)
- Type 2 reactions involve antibody-mediated cytotoxic cellular injury that may result in various cytopenias. (Interpretation: A soldier of your immune system targets an undesirable cell, then attacks it, glues itself to the enemy cell, then destroys it. Cytopenia means the attack is now destroying even your red blood cells.)
- Type 3 and type 4 hypersensitivity results in the formation of antigen–antibody immune complexes or a delayed T cell-mediated reaction, respectively, either of which may progress to the life-threatening Stevens-Johnson syndrome or toxic epidermal necrolysis. (Interpretation: Your immune system goes into tornado mode and starts destroying anything that it thinks could possibly be an enemy cell, even to the point of killing the host body.)
- Top
- I believe the conclusion of this study should be challenged. Is it more likely this woman suffered an anaphlyactic response to the Clorsulon, possibly exacerbated by an eosinophil storm, described as a DRESS event?
- My Question: Because Clorsulon is a sulfonamide, is it possible a hypersensitivity reaction was triggered?
- Yes, because Clorsulon is a sulfonamide, it is possible that a hypersensitivity reaction could be triggered. Sulfonamide antibiotics are known to cause a variety of hypersensitivity reactions, ranging from benign rashes to severe cutaneous adverse reactions like Stevens-Johnson syndrome and toxic epidermal necrolysis. The potential for hypersensitivity reactions with sulfonamides is well-documented, with an incidence of adverse reactions in approximately 3% of the general population, and much higher in patients with conditions like HIV. While Clorsulon is primarily used as an antiparasitic agent, its sulfonamide structure means it shares common characteristics with sulfonamide antimicrobials, which could theoretically lead to cross-reactivity in individuals with a history of sulfonamide hypersensitivity. However, specific data on Clorsulon's hypersensitivity potential might be less extensive since it is not as widely used or studied as some other sulfonamide drugs.nih-1, sci-1
- Top
- Treatment with topical ivermectin 10 mg/g (1%) cream once daily resulted in an almost complete clinical resolution after 15 days [Figure - 4], but direct microscopy was still positive. Maintenance of the treatment for 2 months led to clinical and direct microscopic clearance at follow-up visit, with no relapses in the following 6 months.
- Top
- Ivermectin promotes wound healing partly through modulation of the inflammatory process and the levels of Transforming Growth Factor-Beta 1 and Vascular Endothelial Growth Factor. Low doses of ivermectin cream have the potential to be used in treating wounds with minimal scar tissue formation.
- Top
- The aims of this study are to determine the prevalence of cattle ringworm in native dairy farms of Diyala governorate, and the distribution of T. verrucosum and treatment of the infection in the bovine by ivermectin
- Results: Statistical analysis reveals significantly less clearance time (50%) when ivermectin is used instead of topical antifungal 1% sulpher ointment in treating the disease. With less time of 13-24 days compared to 30-40 days. The treatment of ringworm disease in cattle with clearance rate of 100% is achieved using Ivermectin.
- Top
- Our results demonstrate that ivermectin, an FDA-approved antiparasitic agent, is effective at inhibiting replication of several HAdV types in vitro.
- Top
- Ivermectin is a putative (generally accepted as) antiviral compound.
- My question: If that is true why doesn't the medical industry act like it?
- Top
- The first paragraph states "Neither drug is an anti-viral medication", which according to several studies, is not true41.
- Top
- Ivermectin as a treatment for SARS-CoV-2 in humans has already been approved in a number of states and countries, including the Republic of Peru [44] and Northeastern Beni region of Bolivia [45]. Importantly, close to 70 trials worldwide are currently testing the clinical benefit of ivermectin to treat or prevent SARS-CoV-2 (see Table 3)
- Top
- Ivermectin as a treatment for SARS-CoV-2 in humans has already been approved in a number of states and countries, including the Republic of Peru [44] and Northeastern Beni region of Bolivia [45]. Importantly, close to 70 trials worldwide are currently testing the clinical benefit of ivermectin to treat or prevent SARS-CoV-2 (see Table 3);
- What does "host-directed" really mean? It means the therapy (in this case, Ivermectin) is directed at the host (you), in order to change the environment in which a virus in located. This makes the host an inhospitable place to live and inhibits its ability to replicate.
- Starting in 2012, ivermectin’s antiviral properties have been progressively documented towards a number of RNA viruses, including human immunodeficiency virus (HIV)-1, influenza, flaviruses such as dengue and Zika, and most notably, SARS-CoV-2 (COVID-19) 4, 5, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, as well as DNA viruses such as pseudorabies, polyoma and adenoviruses 18, 19, 20
- Top
- Top
- Top
- Top
- Top
- This article is an "Abstract" only, see 55 for the complete article.
- Top
- Top
- Top
- Top
- Top
- Top
- Top
- Ivermectin @ Edenbridge Pharms | ANDA #204154 | TABLET;ORAL | EDENBRIDGE PHARMS
- Ivermectin | ANDA #210019 | CREAM;TOPICAL | TEVA PHARMS USA
- Ivermectin @ Padagis | ANDA #210225 | CREAM;TOPICAL | PADAGIS ISRAEL
- Ivermectin @ Taro | ANDA #210720 | LOTION;TOPICAL | TARO
- Ivermectin @ Teva Pharms USA | ANDA #212485 | LOTION;TOPICAL | TEVA PHARMS USA
- Ivermectin @ Zydus Pharms USA | ANDA #215210 | CREAM;TOPICAL | ZYDUS
- Top
- Our findings demonstrate the anticancer effect of ivermectin in prostate cancer, indicating that its use may be a new therapeutic approach for prostate cancer.
- Herein, we report the pharmacological effects and targets of ivermectin in prostate cancer.
- Ivermectin caused G0/G1 cell cycle arrest, induced cell apoptosis and DNA damage, and decreased androgen receptor (AR) signaling in prostate cancer cells.
- Recently, several studies have explored the potential of ivermectin as a new cancer treatment nih-2,nat-1,nat-2
- In breast cancer, ivermectin decreases p21-activated kinase 1 (PAK1) expression by promoting its degradation and inducing cell autophagy [aacrj-1]
- In ovarian cancer, ivermectin can block the cell cycle and induce cell apoptosis through a Karyopherin-β1 (KPNB1) related mechanism [pnas-1].
- In leukemia, ivermectin preferentially kills leukemia cells at low concentrations by increasing the influx of chloride ions into cells, which triggers plasma membrane hyperpolarization and reactive oxygen species (ROS) production [cas-1]
- Top
- Top
- This study indicate that ivermectin may have therapeutic benefits for IPF, likely due to its ability to reduce inflammation and mitigate oxidative stress-induced toxicity.
- Top
- Top
- (1) FDA’s Twitter, LinkedIn, and Facebook posts from August 21, 2021 (ECF No. 12, Exs. 4, 5), that read, “You are not a horse. You are not a cow. Seriously, y’all. Stop it.”;
- (2) FDA’s Instagram post from August 21, 2021 (ECF No. 12, Ex. 6), that reads, “You are not a horse. Stop it with the #ivermectin. It’s not authorized for treating #COVID.”
- (3) FDA’s Twitter post from April 26, 2022 (ECF No. 12, Ex. 7), that reads, “Hold your horses, y’all. Ivermectin may be trending, but it still isn’t authorized or approved to treat COVID-19.”
- (4) all other social media posts on FDA accounts that link to Why You Should Not Use Ivermectin to Treat or Prevent COVID-19 (ECF No. 12, Ex. 1).
- Top
- IVM not only has strong effects on parasites but also has potential antiviral effects. IVM can inhibit the replication of flavivirusCDC-1 by targeting the NS3 helicase
- Top
- Objective: To measure the population size and fecundity (ability to reproduce) of the common bed bug after feeding it with the blood obtained from human subjects who have consumed a single dose of ivermectin.
- Conclusions: There were significant reductions in bed bug population size and fecundity in insects that fed on blood obtained from human study subjects up to 96 hours after they have consumed a single oral dose of ivermectin.
- Top
- Ivermectin (IVM) has been proposed as a new tool for malaria control as it is toxic on vectors feeding on treated humans or cattle.
- IVM may have a direct mosquitocidal effect when applied on bed nets or sprayed walls.
- The potential for IVM application as a new insecticide for long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) was tested in this proof-of-concept study in a laboratory and semi-field environment.
- The results showed a direct mosquitocidal effect of IVM on this mosquito strain as all mosquitoes died by 24 h after exposure to IVM.
- Top
- Ivermectin is also highly effective against the ixodid tick Rhipicephalus (Boophilus) microplus, a cattle parasites in the tropics and subtropics.
- Top
- Usual safe medication doses of Ivermectin drug in human beings is 0.2–0.4 mg/kg.
- Top
- Subjects received oral doses of 30 to 120 mg (333 to 2000 mcg/kg) ivermectin in a fasted state or 30 mg (333 to 600 mcg/kg) ivermectin following a standard high-fat (48.6 g of fat) meal
- Administration of 30 mg ivermectin following a high-fat meal resulted in an approximate 2.5-fold increase in bioavailability relative to administration of 30 mg ivermectin in the fasted state.
- Significant lethality was observed in mice and rats after single oral doses of 25 to 50 mg/kg and 40 to 50 mg/kg, respectively. No significant lethality was observed in dogs after single oral doses of up to 10 mg/kgNote: These are more than 100 times the recommended dose.
- Top
- After clinical manifestations (of demodex mites), the mites may be temporarily eradicated with topical insecticides, especially crotamiton cream, permethrin cream, and also with topical or systemic metronidazole. In severe cases, such as those with HIV infection, oral ivermectin may be recommended.
- Colonization of the skin with Demodex could be a reflection of immune response of the host to organism.
- Top
- Drug repositioning( SD1)Drug repurposing (DR), also known as drug repositioning or re-profiling, is an approach to identify new therapeutic indications (other than original indications) of an already approved drug. is defined as a process to identify a new application for drugs.
- In 2018 there were 18.1 million new cancer cases. There were also 9.6 million cancer deaths that year.
- The development of a new drug takes 10–15 years, and the success rate is negligible (approximately 2%).
- There are seven classes of modern anthelmintics
- Ivermectin, as a member of MLs, is metabolized in the liver by the cytochrome P450 system. The blood concentration of the drug reaches the peak 4 h after administration, and after that, the concentration decreases slowly. The metabolites concentration in the blood increases for a longer time when it is compared with the parent compound, which can be resulted from the heterohepatic recycling. The drug can be detected in various sites, including the nodules, and subcutaneous fascia. A single oral dose (12 mg) of ivermectin reaches the peak 8 h after administration in various sites, such as sebum, and forehead, and starts to decrease after 24 h
- Top
- Ivermectin, a US Food and Drug Administration-approved anti-parasitic agent, was found to inhibit severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication in vitro.
- A randomized, double-blind, placebo-controlled trial was conducted to determine the rapidity of viral clearance and safety of ivermectin among adult SARS-CoV-2 patients.
- The trial included 72 hospitalized patients in Dhaka, Bangladesh, who were assigned to one of three groups:
- Oral ivermectin alone (12 mg once daily12 mg once daily is considered the minimal effective prophylactic dose for a person who weighs 133 pounds (see the dosage calculator on this page). Dosage Calculator for 5 days)
- Oral ivermectin in combination with doxycycline (12 mg ivermectin single dose and 200 mg doxycycline on day 1, followed by 100 mg every 12 h for the next 4 days)
- A placebo control group.
- Clinical symptoms of fever, cough, and sore throat were comparable among the three groups.
- Virological clearance was earlier in the 5-day ivermectin treatment arm when compared to the placebo group (9.7 days vs 12.7 days; p = 0.02), but this was not the case for the ivermectin + doxycycline arm (11.5 days; p = 0.27).
- There were no severe adverse drug events recorded in the study.
- A 5-day course of ivermectin was found to be safe and effective in treating adult patients with mild COVID-19.
- Top
- The antiparasitic drug, ivermectin (IVM), exerts anticancer activities in diverse cancer types. However, its anticancer activity against cholangiocarcinoma (CCA), especially the drug-resistant phenotype, has not yet been explored.
- IVM was tested for its anticancer activity against gemcitabine-sensitive (KKU214) and gemcitabine-resistant (KKU214GemR) CCA cell lines.
- IVM treatment inhibited cell proliferation and colony formation of both KKU214 and KKU214GemR in a dose- and time-dependent manner.
- Top
- Ivermectin, an FDA-approved anti-parasitic drug, effectively inhibits infection with hepatitis E virus (HEV) genotypes 1 and 3 in a range of cell culture models, including hepatic and extrahepatic cells.
- Long-term treatment showed no clear evidence of the development of drug resistance.
- Gene silencing of importin-α1, a cellular target of ivermectin and a key member of the host nuclear transport complex, inhibited viral replication and largely abolished the anti-HEV effect of ivermectin. What does this mean?The statement indicates that importin-α1 is vital for HEV replication. Ivermectin targets this protein to inhibit viral replication. However, if importin-α1 is silenced (its expression is reduced or eliminated), HEV replication decreases, but so does ivermectin's ability to fight the virus because it can no longer interact with this key protein in its mechanism of action.
- Top
- Infection with yellow fever virus (YFV), the prototypic mosquito-borne flavivirus, causes severe febrile disease with haemorrhage, multi-organ failure and a high mortality.
- Ivermectin, a broadly used anti-helminthic drug, proved to be a highly potent inhibitor of YFV replication (EC50 values in the sub-nanomolar range).
- Moreover, ivermectin inhibited, although less efficiently, the replication of several other flaviviruses, i.e. dengue fever, Japanese encephalitis and tick-borne encephalitis viruses.
- Considering that this well tolerated drug has been licensed for >20years for the treatment of parasitic infections in man, our results provide the prospect of the first specific anti-flavivirus therapy by the off-label use of ivermectin (patent application EP2010/065880).
- Ivermectin has not been approved by the FDA for use on flaviviruses. Ivermectin is FDA-approved for certain parasitic infections in humans, including strongyloidiasis and onchocerciasis, and for various external parasites like head lice when used topically. However, it has not received FDA approval for treating or preventing infections caused by flaviviruses, which include viruses like Dengue, Yellow Fever, West Nile, and Zika.
Several studies have explored ivermectin's potential antiviral effects against flaviviruses:
- In vitro studies have shown that ivermectin can inhibit the replication of flaviviruses like Dengue, Yellow Fever, and West Nile by specifically targeting their NS3 helicase activity, suggesting potential antiviral effects.
- Despite these findings, there's a lack of clinical data supporting ivermectin's efficacy against flaviviruses in human patients, and the FDA has not authorized or approved ivermectin for this use.
- Top
- These studies provided no evidence that fenbendazole would have value in cancer therapy, but suggested that this general class of compounds merits further investigation.
- Top
- We conducted a dose–response meta-analysis to elucidate the relationship. PubMed and EMBASE were searched for eligible studies up to July 15, 2018.
- Seven eligible cohort studies with 7808 participants were included. The results indicated that higher vitamin D level could reduce the risk of death among prostate cancer patients.
- This meta-analysis suggested that higher 25-hydroxyvitamin D level was associated with a reduction of mortality in prostate cancer patients and vitamin D is an important protective factor in the progression and prognosis of prostate cancer.
- Further dose–response analysis showed that every 20 nmol/L increment in 25-hydroxyvitamin D level was associated with a 9% lower risk of all-cause mortality and prostate cancer-specific mortality.
- Top
- Strongyloidiasis is a gut infection with Strongyloides stercoralis which is common world wide. Chronic infection usually causes a skin rash, vomiting, diarrhoea or constipation, and respiratory problems, and it can be fatal in people with immune deficiency. It may be treated with ivermectin or albendazole or thiabendazole.
- We included seven trials, enrolling 1147 participants, conducted between 1994 and 2011 in different locations (Africa, Southeast Asia, America and Europe).
- Authors' conclusions: Ivermectin results in more people cured than albendazole, and is at least as well tolerated. In trials of ivermectin with thiabendazole, parasitological cure is similar but there are more adverse events with thiabendazole.
- Top
- Fenbendazole is a benzimidazole anthelmintic agent commonly used to treat animal parasitic infections
- In humans, other benzimidazoles, such as mebendazole and albendazole, are used as antiparasitic agents
- Since fenbendazole is not currently approved by the FDA or EMA, its pharmacokinetics and safety in humans have yet to be well-documented in medical literature
- Despite this, insights can be drawn from existing in vitro and in vivo animal studies on its pharmacokinetics
- Given the low cost of fenbendazole, its high safety profile, accessibility, and unique anti-proliferative activities, fenbendazole would be the preferred benzimidazole compound to treat cancer
- To ensure patient safety in the repurposing use of fenbendazole, it is crucial to perform clinical trials to assess its potential anticancer effects, optimal doses, therapeutic regimen, and tolerance profiles
- This review focuses on the pharmacokinetics of orally administered fenbendazole and its promising anticancer biological activities, such as inhibiting glycolysis, down-regulating glucose uptake, inducing oxidative stress, and enhancing apoptosis in published experimental studies
- Additionally, we evaluated the toxicity profile of fenbendazole and discussed possibilities for improving the bioavailability of the drug, enhancing its efficacy, and reducing potential toxicity
- Top
- Repurposing approved non-antitumor drugs is a promising and affordable strategy in drug discovery to identify new therapeutic uses different from the original medical indication that may help increase the number of possible, effective anticancer drugs
- The use of drugs in ways other than their original FDA-approved indications could offer novel avenues such as bypassing the chemoresistance and recurrence seen with conventional therapy and treatment; moreover, it can offer a safe and economic strategy for combination therapy
- Recent works have demonstrated the anticancer properties of the FDA-approved drug Mebendazole
- This synthetic benzimidazole proved effective against a broad spectrum of intestinal Helminthiasis
- Mebendazole can penetrate the blood-brain barrier and has been shown to inhibit the malignant progression of glioma by targeting signaling pathways related to cell proliferation, apoptosis, or invasion/migration, or by increasing the sensitivity of glioma cells to conventional chemotherapy or radiotherapy
- Moreover, several preclinical models and ongoing clinical trials explore the efficacy of Mebendazole in multiple cancers, including acute myeloid leukemia, brain cancer, oropharyngeal squamous cell carcinoma, breast cancer, gastrointestinal cancer, lung carcinoma, adrenocortical carcinoma, prostate cancer, and head and neck cancer
- The present review summarizes central literature regarding the anticancer effects of MBZ in cancer cell lines, animal tumor models, and clinical trials to suggest possible strategies for safe and economical combinations of anticancer therapies in brain cancer
- Mebendazole might be an excellent candidate for the treatment of brain tumors because of its efficacy both when used as monotherapy and in combination as an enhancement to standard chemotherapeutics and radiotherapy, due to its effectiveness on tumor angiogenesis inhibition, cell cycle arrest, apoptosis induction, and targeting of critical pathways involved in cancer such as Hedgehog signaling
- Therefore, attention to MBZ repurposing has recently increased because of its potential therapeutic versatility and significant clinical implications, such as reducing medical care costs and optimizing existing therapies
- Using new treatments is essential, particularly when current therapeutics for patients with brain cancer fail
- Top
- Top
- The small molecule macrocyclic lactone ivermectin, approved by the US Food and Drug Administration for parasitic infections, has received renewed attention in the last eight years due to its apparent exciting potential as an antiviral
- It was identified in a high-throughput chemical screen as inhibiting recognition of the nuclear localizing Human Immunodeficiency Virus-1 (HIV-1) integrase protein by the host heterodimeric importin (IMP) α/β1 complex, and has since been shown to bind directly to IMPα to induce conformational changes that prevent its normal function in mediating nuclear import of key viral and host proteins
- Excitingly, cell culture experiments show robust antiviral action towards HIV-1, dengue virus (DENV), Zika virus, West Nile virus, Venezuelan equine encephalitis virus, Chikungunya virus, Pseudorabies virus, adenovirus, and SARS-CoV-2 (COVID-19)
- Phase III human clinical trials have been completed for DENV, with >50 trials currently in progress worldwide for SARS-CoV-2
- This mini-review discusses the case for ivermectin as a host-directed broad-spectrum antiviral agent for a range of viruses, including SARS-CoV-2
- preliminary results from recently completed study NCT04422561 (Table 2, no. 22), that examines asymptomatic family close contacts of confirmed COVID patients, show that two doses of ivermectin 72 h apart result in only 7.4% of 203 subjects reporting symptoms of SARS-CoV-2 infection, in stark contrast to control untreated subjects, of whom 58.4% reported symptoms, underlining ivermectin’s potential as a prophylactic.
- Top
- The use of albendazole and mebendazole, i.e., benzimidazole broad-spectrum anthelmintics, in treatment of parasitic infections, as well as cancers, is briefly reviewed.
- These drugs are known to block the microtubule systems of parasites and mammalian cells leading to inhibition of glucose uptake and transport and finally cell death.
- Eventually they exhibit ovicidal, larvicidal, and vermicidal effects on parasites, and tumoricidal effects on hosts.
- Albendazole and mebendazole are most frequently prescribed for treatment of intestinal nematode infections (ascariasis, hookworm infections, trichuriasis, strongyloidiasis, and enterobiasis) and can also be used for intestinal tapeworm infections (taeniases and hymenolepiasis). However, these drugs also exhibit considerable therapeutic effects against tissue nematode/cestode infections (visceral, ocular, neural, and cutaneous larva migrans, anisakiasis, trichinosis, hepatic and intestinal capillariasis, angiostrongyliasis, gnathostomiasis, gongylonemiasis, thelaziasis, dracunculiasis, cerebral and subcutaneous cysticercosis, and echinococcosis).
- Albendazole is also used for treatment of filarial infections (lymphatic filariasis, onchocerciasis, loiasis, mansonellosis, and dirofilariasis) alone or in combination with other drugs, such as ivermectin or diethylcarbamazine.
- Albendazole was tried even for treatment of trematode (fascioliasis, clonorchiasis, opisthorchiasis, and intestinal fluke infections) and protozoan infections (giardiasis, vaginal trichomoniasis, cryptosporidiosis, and microsporidiosis).
- These drugs are generally safe with few side effects; however, when they are used for prolonged time (>14–28 days) or even only 1 time, liver toxicity and other side reactions may occur.
- In hookworms, Trichuris trichiura, possibly Ascaris lumbricoides, Wuchereria bancrofti, and Giardia sp., there are emerging issues of drug resistance.
- It is of particular note that albendazole and mebendazole have been repositioned as promising anti-cancer drugs. These drugs have been shown to be active in vitro and in vivo (animals) against liver, lung, ovary, prostate, colorectal, breast, head and neck cancers, and melanoma.
- Two clinical reports for albendazole and 2 case reports for mebendazole have revealed promising effects of these drugs in human patients having variable types of cancers. However, because of the toxicity of albendazole, for example, neutropenia due to myelosuppression, if high doses are used for a prolonged time, mebendazole is currently more popularly used than albendazole in anti-cancer clinical trials.
- Top
- No abstract is available. Must have subscription to Leukemia & Lymphoma Volume 61, 2020 - Issue 10
- Top
- The mitochondrial-stem cell connection (MSCC) theory suggests that cancer originates from chronic oxidative phosphorylation (OxPhos)( SA1)Phosphorylation is the addition of a phosphoryl (PO3) group to a molecule. In biological systems, this reaction is vital for the cellular storage and transfer of free energy using energy carrier molecules. insufficiency in stem cells
- This OxPhos insufficiency leads to the formation of cancer stem cells (CSCs) and abnormal energy metabolism, ultimately resulting in malignancy
- This concept integrates two well-established theories: the cancer stem cell theory and the metabolic theory
- Drawing on insights from molecular biology, pharmacology, and clinical studies, this manuscript introduces a hybrid orthomolecular protocol targeting the MSCC
- The protocol includes 7 therapeutic recommendations, consisting of orthomolecules, drugs, and additional therapies
- The aim of this hybrid orthomolecular protocol is to achieve additive and synergistic effects to enhance OxPhos, inhibit the primary fuels of cancer cells (glucose and glutamine), target CSCs and metastasis
- Thus, numerous experiments suggest that targeting MSCC could be a potential therapeutic approach for cancer treatment
- In order to grow and survive, cancer cells require the primary fuels glucose and glutamine to compensate for OxPhos insufficiency. The respiratory impairment induces overexpression of oncogenes and inactivation of tumor-suppressor genes, which contribute to abnormal energy metabolism in cancer. To date, no evidence has demonstrated the growth of any tumor cells, including CSCs, occurs with the deprivation of fermentable fuels (glucose, pyruvate, or glutamine) (Lee, et al., 2024; Liao, et al., 2017; Holm, et al., 1995; Mathews, et al., 2014; Pastò, et al., 2014).
- Vitamin C demonstrates cytotoxic effects on cancer cells both in vitro and in vivo (Fan, et al., 2023).
- Vitamin D has shown anti-cancer effects in vitro and in vivo for almost all cancer types (Chakraborti, 2011; Seraphin, et al., 2023).
- Zinc supplementation has been recommended as a possible adjunctive treatment for cancer. (Costello & Franklin, 2017; Hoppe, et al., 2021)
- Ivermectin has anti-cancer properties and induces autophagy and apoptosis of cancer cells (Liu, et al., 2020).
- Top
- Purpose: Triple-negative breast cancer (TNBC) often metastasizes to the central nervous system (CNS) and has the highest propensity among breast cancer subtypes to develop leptomeningeal disease (LMD). LMD is a spread of cancer into leptomeningeal space that speeds up the disease progression and severely aggravates the prognosis. LMD has limited treatment options. We sought to test whether the common anti-helminthic drug mebendazole (MBZ) may be effective against murine TNBC LMD.
- Conclusions: We demonstrated that MBZ is a safe and effective oral agent in an animal model of TNBC CNS metastasis. Our findings are concordant with previous efforts involving MBZ and CNS pathology and support the drug's potential utility to slow down leptomeningeal spread.
- Top
- Background: Leptomeningeal metastasis (LM) is a rare complication of prostate cancer (PCa) that involves spread of malignant disease to the meninges and carries a poor prognosis. This review aimed to assess the clinical findings, treatments, and outcomes of LM cases reported in the literature.
- Results: We included 41 patients with a mean age (±SD, range) at diagnosis of 67.5 (8.4, 46-87) years. The most common presenting symptoms were headache (32%), leg weakness (24%), nausea (22%), vomiting (20%), vision complaints (17%), sensory abnormalities (17%), back pain (12%), and confusion (12%). The median time from initial PCa to LM diagnosis was 36 months (range 0-252) and was reported in 37 patients (90%). The median time of survival from LM to death was 1.7 months (range 4 days – 14 months) and was reported in 28 patients (68%). Four patients (13%) were still alive when the reports were written, including one who was alive at 16 months and another at 252 months. The median serum PSA (±SD, range) at time of diagnosis was 137.5 (507.9, 3.1-1500) ng/mL. The most common diagnostic tests were a combination of neuroimaging and CSF analysis (41%), neuroimaging only (32%), and autopsy only (12%). The most common imaging modalities used to aid diagnosis were MRI (65%) and CT (32%). CSF cytology was positive for malignant cells in 55% of 22 cases (54%) that reported cytology results. Thirty-four cases (83%) reported treatments, and the most common treatments were radiation (56%), steroids (32%), surgery (26%), hormonal therapy (21%), and chemotherapy (21%).
- Conclusions: LM is expected to become more prevalent with the overall increasing incidence of PCa with therapeutic advancements, yet it remains difficult to diagnose and treat. The findings of this review suggest that neurological symptoms in patients with advanced PCa should prompt providers to obtain neuroimaging to investigate possible LM.
- The "greenish" notes are my thoughts, NOT PART OF THE STUDY.
- Question: Since Menbendazole is effective against triple-negative breast cancer CNS metastasis, might it be effective against LM? (see reference #82)
- Top
- Introduction: LUAD (Lung adenocarcinoma), the most common subtype of lung carcinoma and one of the highest incidences and mortality cancers in the world remains still a substantial treatment challenge. . . . Though ivermectin has been shown to be effective against a variety of cancers, however, there is few available data reporting the antitumor effects of ivermectin in LUAD.
- Results: Ivermectin treatment strikingly impeded the colony formation, and the viability of the cell, along with cell proliferation, and caused the apoptosis and enhanced autophagy flux in LUAD cells. In addition, ivermectin-induced nonprotective autophagy was confirmed by treating LUAD cells with 3-MA, an autophagy inhibitor. Mechanistically, we found that ivermectin inhibited PAK1 protein expression in LUAD cells and we confirmed that overexpression of PAK1 substantially inhibited ivermectin-induced autophagy in LUAD cells. Based on TCGA and GEO databases, PAK1 was highly expressed in LUAD tissues as compared with normal tissues. Furthermore, LUAD patients with high PAK1 level have poor overall survival. Finally, in vivo experiments revealed that ivermectin efficiently suppressed the cellular growth of LUAD among nude mice.
- Conclusion: This study not only revealed the mechanism of ivermectin inhibited the growth of LUAD but also supported an important theoretical basis for the development of ivermectin during the therapy for LUAD.
- Top
- Lung cancer's mortality is among the highest compared to other cancers globally. However, a recent study has shown that ivermectin, an antiparasitic drug, may have a promising anticancer effect on lung cancer.
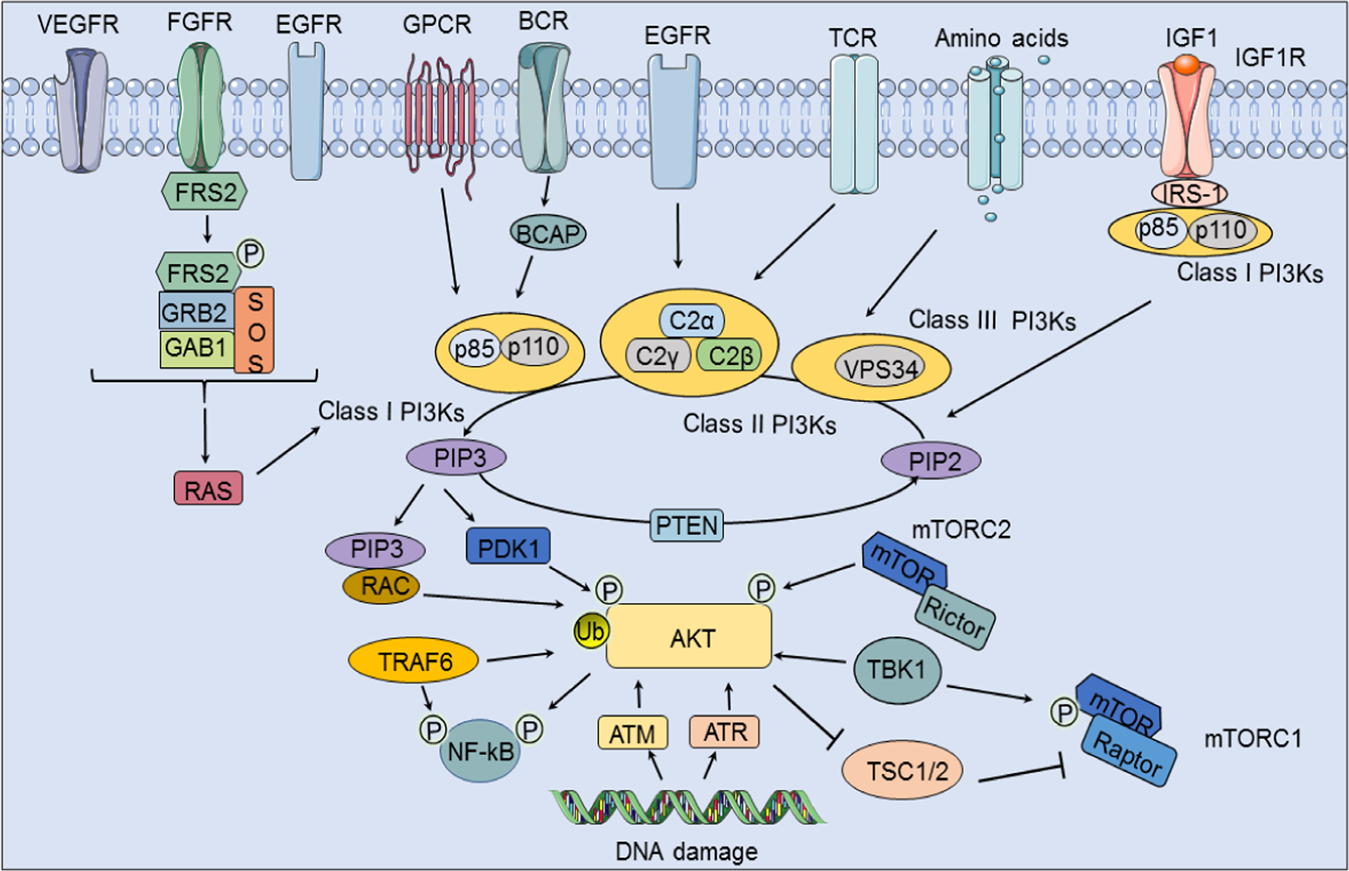
- The present study aimed to investigate the impact of ivermectin on EGFR.3/PI3K4/AKT5/mTOR6 signaling pathway in NSCLC.7
- Mice were divided into four groups;
- normal;
- oral ivermectin alone (5 mg/kg) daily;
- NSCLC was induced by urethane (1.5 g/kg, i.p.) at days one and sixty;
- NSCLC group treated with ivermectin.
- The effect of ivermectin on macroscopic, microscopic, and lung index was assessed.
- The antitumor and antiproliferative effects of ivermectin were investigated by CYFRA 21-1 level and Ki-67, respectively.
- IHC determined the molecular expression of EGFR8, while phosphorylated PI3K, AKT, and mTOR were quantified by Western blotting assay.
- ELISA assay of active caspase 3, Bcl-29, and BAX10 was used to assess the apoptotic effect of ivermectin.
- Finally, VEGF11 lung content was measured.
- Findings showed that ivermectin improved macro and microscopic pathological changes.
- Ivermectin induced cytotoxic effect as indicated by CYFRA 21-1 suppression besides enhancing BAX/Bcl-2 ratio and active caspase 3.
- The immunoexpression of Ki-67 and EGFR declined.
- Ivermectin remarkably reduced p-PI3K, p-AKT, p-mTOR, and VEGF expressions.
- Overall, the study proposes ivermectin as a promising drug for lung cancer through its orchestral regulation of EGFR/PI3K/AKT/mTOR/VEGF signaling.
- Top
- Introduction: Mebendazole, a well-known anti-helminthic drug in wide clinical use, has anti-cancer properties that have been elucidated in a broad range of pre-clinical studies across a number of different cancer types. MBZ is a broad-spectrum benzimidazole anti-helminthic drug, in the same class as albendazole, flubendazole, oxfendazole, and others. It is commonly prescribed to treat a range of parasitical worm infections, including threadworm, tapeworms, roundworms, and other nematode and trematode infections in humans and domestic animals.
- Availability: MBZ is available as a generic drug; common trade names have included Vermox (Janssen Pharamceutica) and Ovex (McNeil Products Ltd) in the US and Europe. It is generally available over the counter in European countries, but the last US manufacturer, Teva Pharmaceuticals, ceased production at the end of 2011, although the drug retains US Food and Drug Administration (FDA) approval.
- Dosage: For human use, the most common formulation of MBZ is as 100 mg chewable tablets. The dosage varies according to the type of helminthic infection being treated. Pinworms are treated with a single 100 mg treatment, whereas roundworms or hookworms are treated with 100 mg twice a day for three days. MBZ, along with albendazole, is also used on a long-term basis for the treatment of human cystic and alveolar echinococcosis (also known as hydatid disease). According to the guidelines published by the World Health Organisation (http://whqlib-doc.who.int/bulletin/1996/Vol74-No3/bulletin_1996_74%283%29_231-242.pdf), long-term treatment of cystic echinococcosis using MBZ is at a dosage of 40–50 mg/kg/day for at least 3–6 months. For alveolar echinococcosis, the dose is 40–50 mg/kg/day, with treatment for at least two years, and possibly longer for patients with inoperable disease. Indeed, there are documented cases of treatment periods of ten or more years [1, 2].
- The "greenish" notes are my thoughts, NOT PART OF THE STUDY.
- Regarding availability: This paper speaks to changes in cost. I am currently (as of 04/25/2025) purchasing MBZ Polymorph C 200mg 90 capsules (which are compounded to be Alpha Gal safe) for $165 + $5 shipping.
- Top
- 2016-02-08 Application filed by Johns Hopkins University
- 2018-01-25 Publication of US20180021310A1
- 2019-07-09 Assigned to THE JOHNS HOPKINS UNIVERSITY
- 2021-09-07 Application granted
- 2021-09-07 Publication of US11110079B2 Status Active
- 2036-02-08 Anticipated expiration
- The "greenish" notes are my thoughts, NOT PART OF THE PATENT.
- The patent number for mebendazole, developed and patented by Janssen Pharmaceutica in 1971, is US3657267A. This patent was filed on June 20, 1969, according to Google Patents
- 1969-06-20 Application filed by Janssen Pharmaceutica NV
- 1972-04-18 Application granted & published
- 1989-04-18 Anticipated expiration Current status: Expired
- Top
- In this series of 5 cases, ivermectin 1% cream was highly effective in reducing or eliminating sleeves, which is the primary clinical sign of D. folliculorum infestation of the eyelids.
- A single or double application of ivermectin 1% cream is well tolerated and highly effective in reducing or eliminating the characteristic sleeves associated with Demodex blepharitis.
- Top
- The results showed that ivermectin but not levamisole or albendazole exhibited a potent anti-staphylococcal activity at the concentrations of 6.25 and 12.5 μg/ml against two isolates.
- Top
- Conclusions: These findings suggest that IVM has the potential to be an alternative remedy for treating piroplasmosis.
- Moreover, this study confirmed the absence of B. microti DNA in groups treated with combination chemotherapy of IVM + DA and IVM + AQ 49 days after infection.
- The "greenish" notes are my thoughts, NOT PART OF THE STUDY.
- This study, while interesting, has limited value for me at this time.
- Top
- Originally developed for veterinary and human use against parasitic infections, ivermectin demonstrated significant antitumor effects in our study against tumor cells (Dalton's lymphoma cells).
- A dose-dependent decrease in tumor cell viability was observed following 24-h treatment with ivermectin, with an IC₅₀ value calculated at 10.55 µg/mL.
- In comparison, the standard anticancer drug cisplatin exhibited a slightly higher cytotoxic potency, with an IC₅₀ of 8.32 µg/mL under the same treatment duration.
- The "greenish" notes are my thoughts, NOT PART OF THE STUDY.
- Based on rough pharmacokinetic estimates, achieving a plasma concentration of 10.55 µg/mL would require an impractical and likely unsafe dose of approximately 73.85 mg/kg in humans, assuming 50% bioavailability and a 3.5 L/kg volume of distribution. However, this is a theoretical calculation, and such doses far exceed safe human doses (0.15–0.2 mg/kg for parasitic infections). Without in vivo data from the study or clinical trials, it’s unclear what dose would achieve antitumor effects in humans, and high doses could be toxic. Always consult a medical professional before considering experimental treatments.
- Cisplatin has significant side effects, as it is a potent chemotherapy drug used to treat various cancers (e.g., testicular, ovarian, lung, and lymphoma). This study notes that cisplatin had an IC₅₀ of 8.32 µg/mL against Dalton’s lymphoma cells, indicating higher cytotoxic potency than ivermectin in vitro. However, cisplatin’s clinical use is associated with well-documented side effects due to its toxicity to both cancer and healthy cells.
- Top
- Background: Fenbendazole (FBZ), an inexpensive and widely accessible antiparasitic drug used in veterinary medicine, has garnered growing interest for its potential as an anticancer therapy. Preclinical studies suggest that FBZ exerts its anticancer effects through a wide variety of mechanisms. While FBZ has shown promise both in vitro and in vivo studies, clinical evidence supporting its use and efficacy in treating metastatic cancer is currently limited.
- Case presentations: This report highlights 3 cases of patients with advanced cancer - including breast, prostate, and melanoma. Two patients achieved complete remission, and one achieved near-complete remission after incorporating FBZ into their treatment regimens alongside other therapies (excluding chemotherapy). All three patients tolerated FBZ without any reported adverse effects, and remission was sustained during follow-up periods ranging from 11 months to nearly 3 years.
- Conclusion: FBZ demonstrates potential as a novel promising therapeutic option for repurposing in oncology. Its ability to contribute to tumor regression and achieve disease remission warrants further clinical research to establish its efficacy and optimize its use.
- If there are "greenish" notes, they are my thoughts, NOT PART OF THE STUDY.
- Top
- In addition to being antiparasitic agents, benzimidazole anthelmintics are known to exert anticancer activities, such as the disruption of microtubule polymerization, the induction of apoptosis, cell cycle (G2/M) arrest, anti-angiogenesis, and blockage of glucose transport.
- These antitumorigenic effects even extend to cancer cells resistant to approved therapies and when in combination with conventional therapeutics, enhance anticancer efficacy and hold promise as adjuvants.
- The present review summarizes central literature regarding the anticancer effects of benzimidazole anthelmintics, including albendazole, parbendazole, fenbendazole, mebendazole, oxibendazole, oxfendazole, ricobendazole, and flubendazole in cancer cell lines, animal tumor models, and clinical trials.
- Benzimidazole anthelmintics induce minimal cytotoxicity in normal cells but high cytotoxicity in tumor cells, exerting a cancer cell-specific selectivity.
- If there are "greenish" notes, they are my thoughts, NOT PART OF THE STUDY.
- Top
- Healthy (normal) mice
- Healthy mice given ivermectin only (5 mg/kg by mouth every day)
- Mice given a chemical called urethane (injected twice) to cause lung cancer (this is a common lab model for NSCLC)
- Cancer mice treated with daily ivermectin
- Lungs looked much healthier (fewer tumors, less damage & inflammation)
- Lower levels of a cancer marker called CYFRA 21-1 → cancer cells were being killed
- Triggered cancer cell suicide (apoptosis) by increasing pro-death proteins (BAX & active caspase-3) and decreasing the "don't-die" protein (Bcl-2)
- Slowed cancer cell multiplication (lower Ki-67 marker)
- Turned down a major cancer growth pathway: EGFR → PI3K → AKT → mTOR (reduced their active forms)
- Lowered VEGF → less new blood vessels to feed the tumor (starving it)
- If there are "greenish" notes, they are my thoughts, NOT PART OF THE STUDY.
- Top
- Zika
- Dengue
- Yellow fever
- West Nile
- Hendra
- Newcastle
- Venezuelan equine encephalitis
- Chikungunya
- Semliki Forest
- Sindbis
- Avian influenza A
- Porcine Reproductive and Respiratory Syndrome (PRRSV)
- Human immunodeficiency virus type 1 (HIV-1)
- Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
- Equine herpes type 1
- BK polyomavirus
- Pseudorabies
- Porcine circovirus 2 (PCV2)
- Bovine herpesvirus 1
- Inhibits nuclear import of viral proteins by disrupting the importin α/β1 (IMP α/β1) heterodimer
- Blocks viral replication steps (e.g., protein transport, RNA synthesis, immune evasion)
- Stronger effects when applied early in infection (pre- or early post-infection)
- May also inhibit helicase activity in some flaviviruses
- In vitro: Strong inhibition observed (e.g., ~5000-fold reduction in SARS-CoV-2 viral RNA in Vero cells at 5 µM)
- In vivo (animal models): Reduced viral loads, lesions, or mortality in models for pseudorabies, PCV2, PRRSV, Newcastle, etc. (results variable)
- If there are "greenish" notes, they are my thoughts, NOT PART OF THE STUDY.
- Top
- Albendazole or praziquantel
- It doesn't always kill all the parasites.
- These drugs have been used for over 40 years → some parasites may be becoming resistant or hard to treat.
- Fenbendazole (FBZ)
- Ivermectin (IVM)
- Injected tapeworm cysts (Taenia crassiceps – similar to the human kind) directly into the mice's brains.
- Treated different groups with:
- Fenbendazole regular or nano – 60 mg/kg
- Ivermectin regular or nano – 0.2 mg/kg
- Albendazole (standard drug) – 40 mg/kg
- Saline (salt water) – control group, no treatment
- All the parasite-killing drugs made the cysts start breaking down and die.
- BUT inflammation was MUCH LOWER in the mice treated with:
- Fenbendazole (regular or nano)
- Ivermectin (regular or nano)
- The nano versions looked especially promising – probably because the tiny particles cross the blood-brain barrier better and deliver the drug more steadily to the cysts.
- The standard albendazole group had significantly more inflammation than the fenbendazole & ivermectin groups.
- If there are "greenish" notes, they are my thoughts, NOT PART OF THE STUDY.
We showed that ivermectin administration was associated significantly with lower mortality among patients with COVID-19, particularly in patients with more severe pulmonary involvement.
This study was conducted in healthy adult volunteers in which we compared 3 treatment regimens: the weight-based reference standard versus 2 experimental regimens of fix-dose 18 and 36 mg using 18 mg tablets. All 54 volunteers received the 3 treatments sequentially. The results confirmed that the fixed-dose regimen (both 18 mg and 36 mg) are as safe as the standard dosage and could justify the use of fix dosing regimens rather than the current weight based strategy.
A brief review of the information availabile regarding the pharmacokinetics and interactions of ivermectin in humans.
Here we show that ivermectin also inhibits infection of epithelial cells by the bacterial pathogen, Chlamydia trachomatis, at doses that could be envisioned clinically for sexually-transmitted or ocular infections by Chlamydia.
For use in humans as an anti-helminthic, ivermectin is typically received orally at a dose of 150 µg/kg body weight, and peak plasma concentrations from such dosage reach around 60 nM
The plasma levels for patients, after 5.2 hours, peaked at 52.0 ng/ml. The elimination half life was 35.0 h;Note: This does not match the accepted HL of 18 hours and the area under the plasma concentration curve versus time, 2852 ng.h.ml-1. In healthy volunteers, the plasma ivermectin distribution was similar to that in patients, and both groups showed a tendency for a second rise in plasma concentration of the drug suggestive of enterohepatic recirculation. Ivermectin was detected in tissues obtained from patients. Fat showed the highest and most persistent levels, whilst values for skin, nodular tissues, and worms were comparable. Subcutaneous fascia contained the lowest concentrations.
Survival of malaria transmitting Anopheles mosquitoes is strongly decreased after feeding on humans recently treated with ivermectin. Currently, mass drug administration of ivermectin is under investigation as a potential novel malaria vector control tool to reduce Plasmodium transmission by mosquitoes. A "post-ivermectin effect" has also been reported, in which the survival of mosquitoes remains reduced even after ivermectin is no longer detectable in blood meals.
Ivermectin’s impact on mosquito mortality is directly related to the time there is a lethal concentration in the blood
The longer the drug remains in the blood, the more mosquitoes it will kill or disable. Any increase in duration of mosquitocidal concentrations is expected to contribute to additional mortality. Modelling shows that the time the drug remains in blood above mosquito-killing levels is the parameter that drives impact on transmission [50].
The next curves indicate change in max plasma concentrattion depending on dose, how multiple doses affect efficacy.
This is an Argentinian study published in Biomedicine & Pharmacotherapy Volume 160, April 2023, 114391.
This is an Argentinian study published in Biomedicine & Pharmacotherapy Volume 160, April 2023, 114391.
Abstract: Ivermectin was used for the treatment of a scabies outbreak in a nursing home. Among the 128 residents, 42 presented pruritus or cutaneous lesions and scabies was parasitologically demonstrated in seven patients. All residents were treated with two 12 mg doses of ivermectin given two weeks apart. Ivermectin treatment associated with procedures for environmental disinfection led to the control of the outbreak. In only one case, was there a failure of the treatment. It is concluded that oral ivermectin is an effective and practical therapy for scabies in nursing homes.
After analyzing the results of the different clinical trials it is concluded that the use of ivermectin in combination with doxycycline can prove to be an effective, safe and affordable therapeutic regimen for relieving the cytokine storm of COVID-19 infection.
Two-dose ivermectin prophylaxis at a dose of 300 µg/kg with a gap of 72 hours was associated with a 73% reduction of SARS-CoV-2 infection among healthcare workers for the following month. Chemoprophylaxis has relevance in the containment of pandemic.
A concentration dependent antiviral activity of oral high dose IVM was identified in this pilot trial at a dosing regimen that was well tolerated.
Interpretation: When 600 mcg/kg IVM was administered, antiviral effects were observed, and that dose was well tolerated.
FDA-approved anti-parasite drug ivermectin is also an antibacterial, antiviral, and anticancer agent, which offers more potentiality to improve global public health, and it can effectively inhibit the replication of SARtarget="_blank"S-CoV-2 in vitro
The findings in this study demonstrate the broad-spectrum antiviral property of ivermectin benefiting for COVID-19 treatment in the context of predictive, preventive, and personalized medicine in virus-related diseases.
Finally, we show for the first time that ivermectin can limit infection by the DENV-related West Nile virus at low (µM)
A concentration dependent antiviral activity of oral high dose IVM was identified in this pilot trial at a dosing regimen that was well tolerated.
Interpretation: When 600 mcg/kg IVM was administered, antiviral effects were observed, and that dose was well tolerated.
There is a relative dirth of studies regarding Clorsulon: A site:nih.gov search shows 905 vs 35,800 for ivermectin.
This is a sheep & goat study that speaks to some questions regarding Clorsulon.: After a single dose of 7 mcg/kg, peak plasma consentration @ 14h (goats) 15h (sheep). There's more in the abstract.
It was considered that the absence of burrows, eggs and mites did not eliminate the risk of scabies infestation. Ivermectin (Maruho Co., Ltd., Osaka, Japan) was administered orally as therapeutic administration (200µg/kg twice at two-week intervals) in 6 patients and as a preventive (one dose of 200µg/kg) in 35 individuals.
Sulfonamide antibiotic reactions encompass the entire spectrum of 4 types of hypersensitivity reactions.
Avermectin is a potent broad-spectrum parasiticidal drug, the discovery of which led to the award of the 2015 Nobel Prize in physiology or medicine. Its derivative, ivermectin, has recently been reported to be a potential anticancer agent against colon cancer, ovarian cancer, melanoma, and leukemia. Our recent study demonstrates that ivermectin also exerts a promising anticancer effect for breast cancer as evidenced by marked growth inhibition after 24-h treatment with ivermectin in a range of breast cancer cell lines, with no obvious effects on nontumorigenic human breast cells. The anticancer activity of ivermectin was further confirmed in both mouse subcutaneous xenografts and orthotopic breast cancer models.
Edenbridge detailed specification and usage sheet for Ivermectin.
Case Study: Patient was diagnosed as having crusted scabies with bullous pemphigoid-like eruptions and nail involvement
prescribed oral ivermectin (two doses of 12 mg ivermectin with a 1-week interval) and topical lindane (1%gamma-BHC in petrolatum) for scabies with 5% salicylic acid in plastibase as an additional treatment for the crusted lesions on his soles. He showed remarkable improvement in 2 weeks, and his nails showed complete recovery after 7 weeks of occlusive dressing treatment with 1%gamma-BHC.
The bullous symptoms of this patient were considered to be due to the scabies, because the patient recovered completely after receiving treatment for scabies. Indirect immunofluorescent study is important to distinguish between scabies with blister formation and true bullous pemphigoid.
Question: Does ivermectin, with a maximum targeted dose of 600 µg/kg daily for 6 days, compared with placebo, shorten symptom duration among adult (=30 years) outpatients with symptomatic mild to moderate COVID-19?
Conclusions and Relevance: Among outpatients with mild to moderate COVID-19, treatment with ivermectin, with a maximum targeted dose of 600 µg/kg daily for 6 days, compared with placebo did not improve time to sustained recovery. These findings do not support the use of ivermectin in patients with mild to moderate COVID-19.
Our findings demonstrate the anticancer effect of ivermectin in prostate cancer, indicating that its use may be a new therapeutic approach for prostate cancer.
There are 219 virus species that are known to be able to infect humans.
The first of these to be discovered was yellow fever virus in 1901, and three to four new species are still being found every year.
Report of a rare case of massive orbital myiasis (fly maggots) following recent lid injury, occurring in the empty socket of an elderly lady, who had concurrent scalp pediculosis (lice). The orbital myiasis was effectively treated with the broad-spectrum antiparasitic agent, ivermectin, thus precluding the need for an exploratory surgery. Ivermectin was also effective in managing the concurrent scalp pediculosis.
A single oral dose of ivermectin was generally effective for the treatment of head lice, especially when used in combination with a LiceMeister® comb and when the whole family was treated. About 15% of patients required a second dose 24 h after the first.
Ivermectin has significant antiarthritic properties and can be a novel treatment agent for the management of rheumatoid arthritis patients suffering from strongyloidiasis.
The conclusion of this study begs the question: Why for strongyloidiasis patients and not others?
Unique method of measuring plasma levels of Ivermectin. Describing the dosing usefully i.e. mg/kg and describing the plasma and fecal concentrations usefully i.e. ng/ml.
The study was conducted in a manner which eliminates many biological variables.
Topical ivermectin is an effective treatment for inflammatory papulopustular rosacea in adults. Positive therapeutic effects of ivermectin due to its potential anti-inflammatory properties could be achieved in the other facial dermatoses.
In December 2014, the US Food and Drug Administration approved 1% ivermectin cream for treatment of rosacea in adults while the first European approval was obtained in March 2015.
The study showed that higher neutrophil levels were linked to mortality in tocilizumab-treated patients, while ivermectin showed promise in increasing survival rates.
Study shows that EP has tremendous potential to target metastatic PCa cells and provides new avenues for therapeutic approaches for advanced PCa.
Retire FDA’s Consumer Update entitled, Why You Should Not Use Ivermectin to Treat or Prevent COVID-19, originally posted on March 5, 2021, and revised on September 7, 2021 (ECF No. 12, Ex. 1), while retaining the right to post a revised Consumer Update.
Delete and not republish
Summary of the anticancer mechanism of IVM
| Mechanism | Cancer type | References |
|---|---|---|
| Inhibit Wnt pathway | Breast cancer, Colorectal cancer | [33,41] |
| Inhibit Akt/mTOR pathway | Breast cancer, Ovarian cancer, Glioma | [32,60,64] |
| Inhibit MAPK pathway | Nasopharyngeal carcinomaMelanoma | [69,73] |
| Inhibit YAP1 protein | Gastric cancer, liver cancer | [39,43] |
| Inhibit PAK1 protein | Breast cancer, Ovarian cancer, Nasopharyngeal carcinoma, Melanoma | [32,58,69,73,96] |
| Inhibit HSP27 | Prostate cancer, Lung cancer Colorectal cancer | [50] |
| Induce mitochondrial dysfunction | Renal cell carcinoma, GliomaLeukemia | [48,53,63] |
| Inhibit cancer stem cells | Breast cancer | [95,96] |
| Inhibit p-glycoproteinand MDR protein | Colorectal cancer, Breast cancerLeukemia | [22,104] |
| Activate P2 × 7 receptor | Breast cancer | [37] |
| Inhibit SIN3 domain | Breast cancer | [36] |
| Inhibit DDX23 helicase | Glioma | [66] |
| Activate chloride channels | Leukemia | [51] |
| Increase TFE3 Activity | Melanoma | [74] |
| Inhibit KPNB1 protein | Ovarian cancer | [59] |
Abstract:
In the body, but not the abstract.
Abstract:
Abstract:
Abstract:
Abstract:
Abstract:
My notes to the reader:
Abstract:
Abstract:
Abstract:
Non-study Comments:
This is a U.S. patent US20180021310A1
Abstract:
Non-patent Comments:
Abstract:
Abstract:
Abstract:
My notes to the reader:
Abstract:
My notes to the reader:
Abstract:
My notes to the reader:
Abstract:
My notes to the reader:
Abstract: Summarized using GROK
Ivermectin and Lung Cancer – Easy-to-Understand Summary
From a 2025 mouse study published in the journal Tissue and Cell (August 2025)
Lung cancer (especially non-small cell lung cancer, or NSCLC) is one of the deadliest cancers worldwide. This study tested whether ivermectin — the familiar antiparasitic drug — could help fight it in mice.
How They Did the Experiment
They used four groups of mice:
They examined the lungs in many ways: appearance (naked eye + microscope), tumor markers, cell growth/death signals, and specific proteins.
What They Found – In Simple Terms
Ivermectin helped the cancer mice in several big ways:
Visual: The Key Pathway Ivermectin Targets (EGFR/PI3K/AKT/mTOR)
Here's a clear diagram showing the signaling chain that gets overactive in many lung cancers — ivermectin reduced activity all along this path:

Visual: How Ivermectin Promotes Cancer Cell Death (Apoptosis)
It flips the balance toward cell death by boosting BAX/caspase-3 and reducing Bcl-2:

Bottom Line from the Researchers
• Done only in mice (not humans)
• Used a chemical-induced cancer model
• Animal results don't always work the same in people
• Ivermectin is NOT approved or recommended as a cancer treatment for humans yet
• There are interesting lab/animal studies on ivermectin in cancer, but big human clinical trials for lung cancer are still needed
• Always talk to a doctor/oncologist about real treatments — never self-medicate
This summary is based on the August 2025 study abstract — science moves fast, so stay tuned for more research!
My notes to the reader:
Abstract: Summarized using GROK
Ivermectin: Antiviral Effects Systematic Review Summary
Paper: "Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen" (2020)
Authors: Fatemeh Heidary & Reza Gharebaghi
Journal: The Journal of Antibiotics | PMCID: PMC7290143
Overview
This comprehensive systematic review summarizes in vitro and in vivo studies (spanning ~50 years) on the antiviral properties of ivermectin, a widely used antiparasitic drug with additional antimicrobial, antiviral, and anti-cancer potential.
Ivermectin shows broad-spectrum antiviral activity, particularly against positive-sense single-stranded RNA viruses, and is proposed as a potential candidate for treating viruses including SARS-CoV-2 (COVID-19).
Viruses with Reported Antiviral Effects
RNA Viruses
DNA Viruses
Key Mechanisms of Action
Evidence from Studies
Important Limitations & Conclusions
While preclinical data (in vitro & animal) are promising, effective concentrations in cell studies often exceed safe human plasma levels achievable with standard doses.
In vivo results are inconsistent across models, and no robust human clinical trial data supported antiviral efficacy at the time of publication (2020).
The authors conclude: Clinical trials are necessary to evaluate ivermectin's potential efficacy in humans, including as a complementary regimen for COVID-19 and other viruses.
Note: This review reflects early pandemic-era interest. Subsequent high-quality clinical evidence has generally not supported ivermectin’s efficacy against COVID-19 in humans.
My notes to the reader:
Abstract: Summarized using GROK
Neurocysticercosis (NCC) – A Brain Parasite Infection
Explained Simply from a Recent Research Study
Neurocysticercosis (NCC) is a brain infection caused by baby tapeworms (called cysticerci) from Taenia solium. It is the most common preventable parasitic disease that affects the brain and nervous system worldwide.
How It's Normally Treated (and Why It's Not Perfect)
Doctors usually give drugs that kill parasites:
These are combined with corticosteroids (like prednisone) because when the parasites die, they cause a very strong inflammatory reaction in the brain → swelling, irritation, headaches, seizures, etc.
Problems with current treatment:
What This New Study Tested
Researchers wanted to see if two other parasite-killing drugs (normally used in animals or for other infections) could work better or cause less harm in the brain:
They tested both the normal versions and special nanoformulations (tiny-particle versions that may get into the brain more easily).
How the Experiment Was Done
They used mice as a model:
After treatment, they examined the brains under a microscope to see what happened to the parasites and the brain tissue around them.
Main Results – in Plain Words
Why This Could Be Important
The usual drug (albendazole) kills parasites but often causes a lot of harmful brain inflammation when the parasites die. Fenbendazole and ivermectin (especially the nano versions) seem to kill the parasites while causing much less damaging inflammation. This could lead to safer or better treatments in the future.
Important: This study was done in mice, not people. It's early research showing promising results — not something doctors can use yet. More studies (including human trials) would be needed.
Based on the publicly available abstract of the article from Acta Tropica (2025).
My notes to the reader: